MARK: 73
Student no:
Module : Principles of BiologyDate 19.11.2010
Essay Question:
Briefly explain the process of Protein Synthesis in terms of nucleic acids and, using a specific example, explain how a change in DNA may cause a new phenotypic characteristic?
The Process of Protein Synthesis
The DNA inherited by an organism leads to specific trait by dictating the synthesis of proteins. Proteins are the link between genotype and phenotype. The process by which DNA directs protein synthesis ( gene expression) includes two stages : transcription and translation.(Fig 1.) (Campbell, 2005)
Transcriptionis the synthesis of RNA under the direction of DNA. Both nucleic acids use the same language and the information is simply transcribed or copied form DNA molecule to RNA molecule. The base-pairing rules for DNA synthesis (AT CG GC TA) also guide transcription, but uracil (U) takes the place of thymine (T) in RNA. A gene does not build a protein directly, the bridge between DNA and the protein synthesis is the nucleic acid RNA. The RNA molecule which carries the genetic message from the DNA to the protein synthesizing system is called messenger RNA (mRNA) (Fig 1.) (Campbell, 2005)
Fig 1 . Transcription – Translation – Protein. Gene expression.
Source : Campbell and Reece , Biology 7th Edition 2005 pg 313.
Translation is the process of synthesis of polypeptide which occurs under the direction of mRNA.(Fig.1) (Campbell, 2005)
During this stage, there`s a change in language : the cell must translate the base sequence of an mRNA molecule into the amino acid sequence of a polypeptide. The sites of translation are ribosomes , complex particles that facilitate the orderly linking of amino acids into polypeptide chains.
The genetic code of mRNA is read by tRNA as triplets of nucleotides named codons.The codons (base triplets) in the mRNA are recognized by tRNAs which carry the appropriate amino acid to the translation machinery. (Fig.2)
Each tRNA is specific for one amino acid but many of the tRNAs can recognize more than one
codon. There are 61 possible codons and approximately 50 tRNAs in animal cells.
Three codons (UAG, UAA, and UGA) do not specify an amino acid-tRNA and
thus cause termination of translation. These codons signal the release of the polypeptide chain. The
ribosome then disengages from the mRNA and the subunits dissociate, ready to start the cycle
over again.
Fig.2 Translation of genetical information.
Source : Campbell and Reece , Biology 7th Edition 2005 pg 320.
In a cell lacking a nucleus, Prokaryotic cell , mRNA produced by transcription is immediately translated without additional processing .(Fig 3 .)
Fig 3 .Prokaryotic cell .
Source : Campbell and Reece , Biology 7th Edition 2005 pg.312
The nucleus of a eukaryotic cell provides a separate compartment for transcription. The original RNA transcript, called pre-mRNA, is processed in various ways before leaving the nucleus as mRNA.
(Fig . 4)
Fig4 . Eukaryotic cell.
Source : Campbell and Reece , Biology 7th Edition 2005 pg.312
Sickle Cell Anemia
The most common inherited disorder among people of African descendent is sickle-cell disease, which affects one out of 400 African-Americans. This disease was caused by modifications in DNA structure. The changes in the genetic material of a cell are called mutations. Spontaneous mutations are caused by errors during DNA replication, repair or recombination and can lead to base pair substitution , insertions or deletions. (Campbell, 2005)
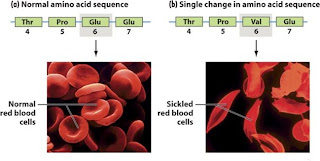
Point mutations are chemical changes in just one base pair of a gene. The change of a single nucleotide in the DNA`s template strand leads to the production of an abnormal protein. Point mutations can affect protein structure and function and therefore observable physical and biochemical characteristics of an organism- changes in phenotype (Fig.6 and Fig.7).
Sickle cell disease is caused by a mutation of a single base pair in the gene that codes for one of the polypeptides of hemolgobin as shown in Fig. 5.
 |
| DNA normal |
 |
| DNA mutant |
The diagram above shows the difference in the synthesis of hemoglobin from normal DNA and from mutant DNA.
A single substitution (T with A), point mutation, has changed the base sequence in the DNA. The base sequence on the mRNA produced in transcription from the mutant template strand of DNA is different from the structure of normal mRNA. As a result a codon on the mutant mRNA is altered. A different amino acid is inserted into the protein chain. The amino acid valine is inserted instead of glutamic acid. The protein synthesised is altered and no longer functions normally. These sequences result in sickle-shaped red blood cells. This blood disorder is known as sickle cell anaemia.
Fig.6 Comparison of physical shape of normal hemoglobine a sickle-cell disease hemoglobine.
Source :http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=iga&part=A1622#A1638
The change of just one amino acid is sometimes enough to alter protein function. This was first shown in 1957 by Vernon Ingram, who studied the globular protein haemolgobin.
Ingram compared hemoglobin A (HbA), the hemoglobin from normal adults, with hemoglobin S (HbS), the protein from people homozygous for the mutant
gene that causes sickle-cell anemia. Ingram found that the fingerprint of HbS differs from that of HbA in only one spot. Sequencing that spot from the two kinds of hemoglobin, Ingram found that only one amino acid in the fragment differs in the two kinds. Apparently, of all the amino acids known to make up a hemoglobin molecule, a substitution of valine for glutamic acid at just one point, position 6 in the β chain, is all that is needed to produce the defective hemoglobin (Fig. 7). Unless patients with HbS receive medical attention, this single error in one amino acid in one protein will hasten their death.
Protein architecture is the key to gene function, the substitution of a amino acid into the polypeptide sequence of a protein leads to new chemical properties that are incompatible with the proper protein architecture at that particular position; in such a case, the mutation will lead to a non-functional protein as shown in fig 7 and 10.
Fig. 7 Comparison of structure and function between normal and sickle-cell hemoglobin.
Source : Campbell and Reece , Biology 7th Edition 2005 pg.84
Fig. 8 Normal hemoglobin
Source: http://www.medicineamigo.com/
Fig.9 Comparison of physical shape.
Source: http://www.bertmiddletonhealth.com/
Normal red blood cells are disc-shaped and move easily through blood vessels.Sickle cells contain abnormal hemoglobin that causes the cells to have a sickle, or crescent, shape. These cells don't move easily through blood vessels. They're stiff and sticky and tend to form clumps and get stuck in the blood vessels. The clumps of sickle cells block blood flow in the blood vessels in the limbs and organs. Blocked blood vessels can cause pain, serious infections, and organ damage.
Fig. 10 Blood flow in a small blood vessel .Comparison between normal and sickle-cells of haemolgobin.
Source: http://www.nhlbi.nih.gov/health/dci/Diseases/Sca/SCA_WhatIs.html
Figure A shows normal red blood cells flowing freely in a blood vessel. The inset image shows a cross-section of a normal red blood cell with normal hemoglobin.
Figure B shows abnormal, sickled red blood cells clumping and blocking blood flow in a blood vessel The inset image shows a cross-section of a sickle cell with abnormal hemoglobin forming abnormal strands.
Red blood cells are made in the spongy marrow inside the large bones of the body. Bone marrow is always making new red blood cells to replace old ones. Normal red blood cells live about 120 days in the bloodstream and then die. In sickle cell anemia, the number of red blood cells is low because sickle cells don't last very long. Sickle cells usually die after only about 10 to 20 days. The bone marrow can't make new red blood cells fast enough to replace the dying ones.
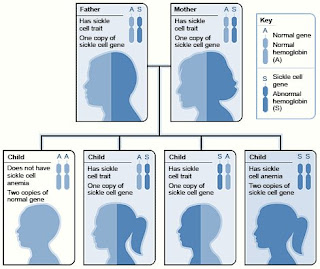
Sickle cell anemia is an inherited, lifelong disease. People who have the disease are born with it. They inherit two copies of the sickle cell gene—one from each parent.
People who inherit a sickle cell gene from one parent and a normal gene from the other parent have a condition called sickle cell trait.
People who have sickle cell trait don't have the disease, but they have one of the genes that cause it. Like people who have sickle cell anemia, people who have sickle cell trait can pass the sickle cell gene on to their children.
Two copies of the sickle cell gene are needed for the body to make the abnormal hemoglobin found in sickle cell anemia.
Fig.11 Example of an Inheritance Pattern for Sickle Cell Trait
Source: http://www.nhlbi.nih.gov/health/dci/Diseases/Sca/SCA_WhatIs.htmlThe image shows how sickle cell genes are inherited. A person inherits two copies of the hemoglobin gene—one from each parent. A normal gene will make normal hemoglobin (A). An abnormal (sickle cell) gene will make abnormal hemoglobin (S).
In individuals who are homozygous for the mutant allele, the sickling of red blood cells caused by the altered hemoglobin produces the multiple symptoms associated with sickle-cell disease (Fig 12 ).
Figure 12 The compounded consequences of one amino acid substitution in hemoglobin to produce sickle-cell anemia.
Source: http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=iga&part=A1622#A1638
Resources
- Campbell et al, Biology, 2005, 7th edition, Pearson Education, Inc., Benjamin Cummings, San Francisco. ISBN: 0-321-26984-5
- http://www.bbc.co.uk/scotland/learning/bitesize/higher/biology/genetics_adaptation/mutations_rev1.shtml (visited on 8 nov 2010)
- http://bertmiddletonhealth.com/health-concerns/sickle-cell/ (visited on 9 nov 2010)
- http://www.medicineamigo.com/disease/Famous-People-With-Blood-Diseases.html
- http://www.nhlbi.nih.gov/health/dci/Diseases/Sca/SCA_WhatIs.html (visited on 8 nov 2010)
- http://www.ncbi.nlm.nih.gov/mapview/maps.cgi?ORG=hum&CHR=11&MAPS=ideogr%5B11pter%3A11qter%5D,loc%5B0.000000%3A142127415.000000%5D&query=e:HBB (visited on 10 nov 2010)









No comments:
Post a Comment